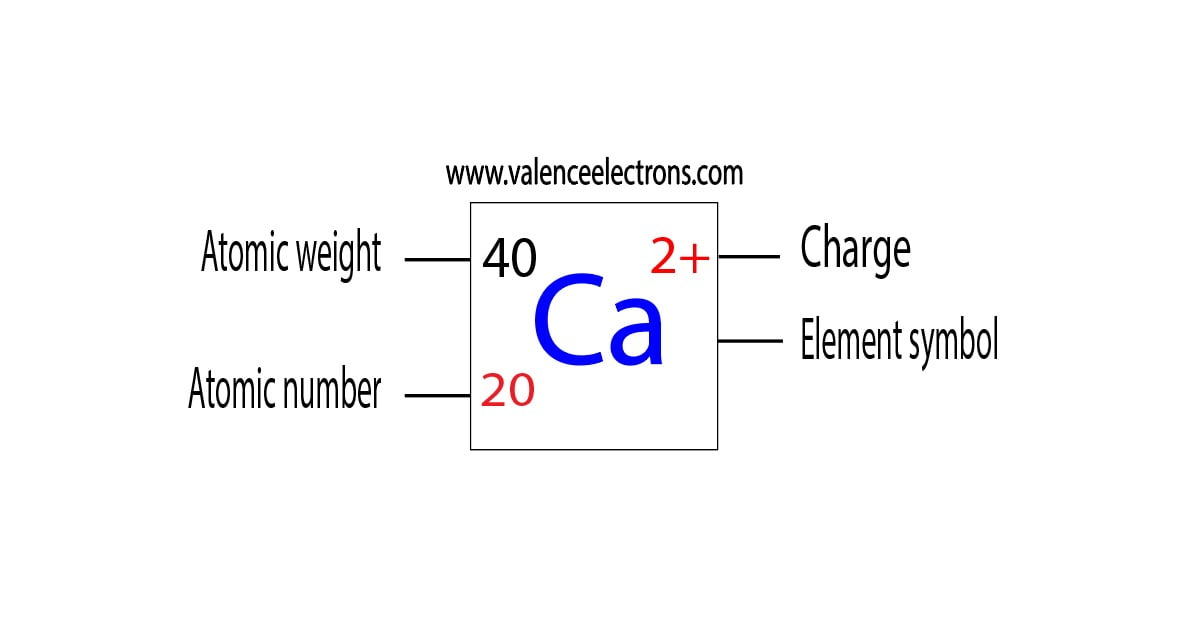
What S The Electron Configuration Of A Ca 2 Ion . We start by filling the 1s subshell with two. in this video we will write the electron configuration for ca2+, the calcium. when a ca atom loses both of its valence electrons, the result is a cation with 18 electrons, a 2+ charge, and an electron. in writing the electron configuration for calcium the first two electrons will go in the 1s orbital. Let’s write the electronic configuration of a calcium atom first. Hence, we can say that both are. the electron configuration for ca 2+ is the same as that for argon, which has 18 electrons. therefore, a calcium atom has 20 electrons. Since 1s can only hold two.
from valenceelectrons.com
therefore, a calcium atom has 20 electrons. the electron configuration for ca 2+ is the same as that for argon, which has 18 electrons. in this video we will write the electron configuration for ca2+, the calcium. when a ca atom loses both of its valence electrons, the result is a cation with 18 electrons, a 2+ charge, and an electron. Hence, we can say that both are. Let’s write the electronic configuration of a calcium atom first. in writing the electron configuration for calcium the first two electrons will go in the 1s orbital. Since 1s can only hold two. We start by filling the 1s subshell with two.
Electron Configuration for Calcium (Ca, Ca2+ ion)
What S The Electron Configuration Of A Ca 2 Ion in this video we will write the electron configuration for ca2+, the calcium. when a ca atom loses both of its valence electrons, the result is a cation with 18 electrons, a 2+ charge, and an electron. in writing the electron configuration for calcium the first two electrons will go in the 1s orbital. in this video we will write the electron configuration for ca2+, the calcium. Since 1s can only hold two. the electron configuration for ca 2+ is the same as that for argon, which has 18 electrons. Hence, we can say that both are. therefore, a calcium atom has 20 electrons. We start by filling the 1s subshell with two. Let’s write the electronic configuration of a calcium atom first.
From wiringlistvoltairean.z14.web.core.windows.net
How To Write Electron Configuration Diagrams What S The Electron Configuration Of A Ca 2 Ion Since 1s can only hold two. therefore, a calcium atom has 20 electrons. in this video we will write the electron configuration for ca2+, the calcium. We start by filling the 1s subshell with two. Hence, we can say that both are. the electron configuration for ca 2+ is the same as that for argon, which has. What S The Electron Configuration Of A Ca 2 Ion.
From www.slideserve.com
PPT How do atoms form ions? PowerPoint Presentation, free download What S The Electron Configuration Of A Ca 2 Ion in this video we will write the electron configuration for ca2+, the calcium. in writing the electron configuration for calcium the first two electrons will go in the 1s orbital. Hence, we can say that both are. therefore, a calcium atom has 20 electrons. We start by filling the 1s subshell with two. Let’s write the electronic. What S The Electron Configuration Of A Ca 2 Ion.
From galvinconanstuart.blogspot.com
Lewis Dot Diagram For Ca General Wiring Diagram What S The Electron Configuration Of A Ca 2 Ion Hence, we can say that both are. therefore, a calcium atom has 20 electrons. Let’s write the electronic configuration of a calcium atom first. the electron configuration for ca 2+ is the same as that for argon, which has 18 electrons. We start by filling the 1s subshell with two. in this video we will write the. What S The Electron Configuration Of A Ca 2 Ion.
From www.slideserve.com
PPT Atomic Structure II PowerPoint Presentation, free download ID What S The Electron Configuration Of A Ca 2 Ion in writing the electron configuration for calcium the first two electrons will go in the 1s orbital. Hence, we can say that both are. the electron configuration for ca 2+ is the same as that for argon, which has 18 electrons. when a ca atom loses both of its valence electrons, the result is a cation with. What S The Electron Configuration Of A Ca 2 Ion.
From vanceknoehampton.blogspot.com
Electronic Configuration of Elements VanceknoeHampton What S The Electron Configuration Of A Ca 2 Ion in writing the electron configuration for calcium the first two electrons will go in the 1s orbital. the electron configuration for ca 2+ is the same as that for argon, which has 18 electrons. when a ca atom loses both of its valence electrons, the result is a cation with 18 electrons, a 2+ charge, and an. What S The Electron Configuration Of A Ca 2 Ion.
From www.sciencephoto.com
Calcium electron configuration Stock Image C029/5027 Science What S The Electron Configuration Of A Ca 2 Ion Hence, we can say that both are. Let’s write the electronic configuration of a calcium atom first. Since 1s can only hold two. therefore, a calcium atom has 20 electrons. in this video we will write the electron configuration for ca2+, the calcium. the electron configuration for ca 2+ is the same as that for argon, which. What S The Electron Configuration Of A Ca 2 Ion.
From homedeso.vercel.app
Iron Periodic Table Electron Configuration What S The Electron Configuration Of A Ca 2 Ion in writing the electron configuration for calcium the first two electrons will go in the 1s orbital. Let’s write the electronic configuration of a calcium atom first. in this video we will write the electron configuration for ca2+, the calcium. therefore, a calcium atom has 20 electrons. We start by filling the 1s subshell with two. . What S The Electron Configuration Of A Ca 2 Ion.
From www.slideserve.com
PPT Chemical Bonding PowerPoint Presentation, free download ID3249254 What S The Electron Configuration Of A Ca 2 Ion the electron configuration for ca 2+ is the same as that for argon, which has 18 electrons. therefore, a calcium atom has 20 electrons. Hence, we can say that both are. Let’s write the electronic configuration of a calcium atom first. when a ca atom loses both of its valence electrons, the result is a cation with. What S The Electron Configuration Of A Ca 2 Ion.
From www.numerade.com
SOLVEDWrite the electron configuration for each ion. What do all of What S The Electron Configuration Of A Ca 2 Ion therefore, a calcium atom has 20 electrons. Hence, we can say that both are. We start by filling the 1s subshell with two. the electron configuration for ca 2+ is the same as that for argon, which has 18 electrons. in this video we will write the electron configuration for ca2+, the calcium. when a ca. What S The Electron Configuration Of A Ca 2 Ion.
From www.youtube.com
Electron Configuration of Ions YouTube What S The Electron Configuration Of A Ca 2 Ion in writing the electron configuration for calcium the first two electrons will go in the 1s orbital. the electron configuration for ca 2+ is the same as that for argon, which has 18 electrons. Hence, we can say that both are. therefore, a calcium atom has 20 electrons. We start by filling the 1s subshell with two.. What S The Electron Configuration Of A Ca 2 Ion.
From www.youtube.com
Electron configurations of ions Atomic structure and properties AP What S The Electron Configuration Of A Ca 2 Ion Since 1s can only hold two. the electron configuration for ca 2+ is the same as that for argon, which has 18 electrons. therefore, a calcium atom has 20 electrons. in writing the electron configuration for calcium the first two electrons will go in the 1s orbital. Hence, we can say that both are. when a. What S The Electron Configuration Of A Ca 2 Ion.
From www.sciencefacts.net
Electron Configuration Definition, Examples, Chart, and Diagram What S The Electron Configuration Of A Ca 2 Ion We start by filling the 1s subshell with two. in this video we will write the electron configuration for ca2+, the calcium. therefore, a calcium atom has 20 electrons. the electron configuration for ca 2+ is the same as that for argon, which has 18 electrons. when a ca atom loses both of its valence electrons,. What S The Electron Configuration Of A Ca 2 Ion.
From www.youtube.com
CHEMISTRY 101 Electron configurations for ions YouTube What S The Electron Configuration Of A Ca 2 Ion Let’s write the electronic configuration of a calcium atom first. in writing the electron configuration for calcium the first two electrons will go in the 1s orbital. Since 1s can only hold two. in this video we will write the electron configuration for ca2+, the calcium. the electron configuration for ca 2+ is the same as that. What S The Electron Configuration Of A Ca 2 Ion.
From www.youtube.com
How to find Protons & Electrons for the Calcium ion (Ca 2+) YouTube What S The Electron Configuration Of A Ca 2 Ion therefore, a calcium atom has 20 electrons. Hence, we can say that both are. in writing the electron configuration for calcium the first two electrons will go in the 1s orbital. Since 1s can only hold two. the electron configuration for ca 2+ is the same as that for argon, which has 18 electrons. in this. What S The Electron Configuration Of A Ca 2 Ion.
From www.numerade.com
SOLVEDWhat is the electron configuration of Ca^2+ ? What is the What S The Electron Configuration Of A Ca 2 Ion in this video we will write the electron configuration for ca2+, the calcium. Let’s write the electronic configuration of a calcium atom first. the electron configuration for ca 2+ is the same as that for argon, which has 18 electrons. Hence, we can say that both are. therefore, a calcium atom has 20 electrons. Since 1s can. What S The Electron Configuration Of A Ca 2 Ion.
From www.youtube.com
6.8 Electron Configurations of Ions YouTube What S The Electron Configuration Of A Ca 2 Ion the electron configuration for ca 2+ is the same as that for argon, which has 18 electrons. in writing the electron configuration for calcium the first two electrons will go in the 1s orbital. Let’s write the electronic configuration of a calcium atom first. when a ca atom loses both of its valence electrons, the result is. What S The Electron Configuration Of A Ca 2 Ion.
From johelpschemistry.blogspot.com
ALevel Chemistry 1.6.1c draw electron configuration diagrams of What S The Electron Configuration Of A Ca 2 Ion therefore, a calcium atom has 20 electrons. in writing the electron configuration for calcium the first two electrons will go in the 1s orbital. Hence, we can say that both are. the electron configuration for ca 2+ is the same as that for argon, which has 18 electrons. when a ca atom loses both of its. What S The Electron Configuration Of A Ca 2 Ion.
From sciencenotes.org
List of Electron Configurations of Elements What S The Electron Configuration Of A Ca 2 Ion Let’s write the electronic configuration of a calcium atom first. Since 1s can only hold two. We start by filling the 1s subshell with two. therefore, a calcium atom has 20 electrons. in writing the electron configuration for calcium the first two electrons will go in the 1s orbital. the electron configuration for ca 2+ is the. What S The Electron Configuration Of A Ca 2 Ion.